Overview and History of Ephedrine
Ephedrine is not an anabolic steroid, but instead belongs to a particular category of compounds known as sympathomimetics (more commonly known as stimulants). It is a sympathomimetic amine that acts upon both the alpha and beta adrenoreceptors. This makes Ephedrine an alpha and beta adrenergic receptor agonist. In contrast to Clenbuterol, another similar sympathomimetic, Clenbuterol is much more selective as it almost exclusively targets the beta-2 adrenoreceptors in the body. Ephedrine is a member of a large family of these sympathomimetic stimulant compounds, which includes others such as: Caffeine, Albuterol, Ephedrine, Dextroamphetamine, Methamphetamine, Cocaine, and many others. It is a very broad category of compounds. All of these compounds, which include Caffeine, Albuterol, Clenbuterol, Ephedrine, Epinephrine, Norepinephrine, and so on and so forth, are all related to one another and could be considered siblings or ‘cousins’ to each other. Because of this they are related in many ways and carry many similarities.
All of the mentioned compounds (stimulants/sympathomimetics) will act upon the nervous system and essentially increase the activity of the CNS (Central Nervous System), among other effects[1]. Ephedrine will also increase the secretion of Norepinephrine (also known as Noradrenaline) in the body, which will further assist to act upon alpha and beta receptors in the body. The actions that Ephedrine has upon the alpha and beta receptors will trigger different effects in different cell types and tissues in the body. For example, much like Clenbuterol, Ephedrine when acting upon the alpha and beta receptors of the cells lining the bronchial pathways, will cause bronchial dilation (the opening of the bronchial pathways). This is especially important for asthma patients, which Ephedrine has been used for in traditional Chinese medicine for hundreds of years[2]. When Ephedrine acts upon the alpha and beta receptors of fat cells, it will initiate the process of lipolysis (fat breakdown) whereby free fatty acids are mobilized and released from the fat cell for consumption by the body as fuel/energy. The actions of Ephedrine upon the CNS will also increase the ability to generate nerve impulses more efficiently, and therefore allow the user to generate stronger and more forceful muscle contractions (often very beneficial for weight training, power lifters, and bodybuilders). As one can easily see, the applications of Ephedrine go far beyond the scope of simple fat loss.
As a fat loss agent, Ephedrine is perhaps on par with Clen in popularity and is perhaps even more commonly known than Clenbuterol. Ephedrine as a compound and medication has been known to man for thousands of years, and is considered a very old medicine. It has been used in traditional Chinese medicine for thousands of years and its use dates back in China to the Han Dynasty[3]. In china it is known as Ma Huang, and was used in its natural form from the Ephedra Sinica (known in China as ‘Ma Huang’) plant, which contains the active compound Ephedrine within it. The synthesis of Ephedrine was performed in 1885 by Japanese chemist Nagi Nagayoshi, and the industrial mass-scale manufacture of Ephedrine in China was underway in the 1920s by the pharmaceutical company Merck[4].
In North America and the United States, Ephedrine has been used for various medical application over several decades, which includes its use as a stimulant, nasal decongestant, appetite suppressant, as well as other applications. While it once was an over-the-counter (OTC) item in the United States, it is now a behind-the-counter (BTC) medication in most states. Contrary to popular belief, the increased controls over Ephedrine throughout the 2000s was not because of its responsibility for a handful of deaths in users utilizing it as a fat burning drug, but because Ephedrine serves as a key ingredient in the synthesis of methamphetamines. Therefore, many states have enacted state level laws controlling the sale of Ephedrine. In 2006, for example, federal legislation on Ephedrine was enacted which required records to be kept of Ephedrine sales in the United States due to the increase of underground illicit methamphetamine laboratories and methamphetamine use/addiction. Despite the controls and restrictions, it is still available for purchase in most US states, though various states hold tighter controls over it than others.
Chemical Characteristics of Ephedrine
Ephedrine belongs to the class of sympathomimetic drugs, and is a sympathomimetic amine. It is very closely related to amphetamine and methamphetamine, and holds similar effects on animal physiology, though to a lesser extent in many cases.
Properties of Ephedrine
Ephedrine will exhibit various effects on the body through its ability to directly interact with alpha and beta receptors, as well as its ability to enable the increased secretion of Norepinephrine[5]. The best analogy to use in terms of Ephedrine’s activity in the body would be its comparison to Clenbuterol in relation to a hammer and nails analogy: Clenbuterol’s action of selectively activating Beta-2 receptors is the equivalent of having several nails sticking out of a wooden surface, and a hammer is used to hammer one specific nail on the head, while Ephedrine is the equivalent of using a larger sledgehammer to hit multiple nails on the head to drive them into the wood. Although not a perfect analogy, this explains Ephedrine’s activity with a fair amount of accuracy. Through its activity with the alpha and beta receptors, as described earlier, it will initiate the process of fat loss in fat cells in the body. Its interaction with the same receptor types on other cells, such as the CNS, will increase the ability for additional force generation by skeletal muscles and thereby improve performance in a more short-term immediate sense.
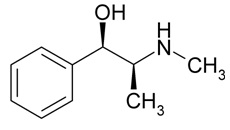 Ephedrine
Ephedrine
Chemical Name: (R*,S*)-2-(methylamino)-1-phenylpropan-1-ol
Molecular Weight: 165.23 g/mol
Formula: C10H15NO
Original Manufacturer: Merck
Half Life: 3 – 6 hours
Detection Time: 5 days
Anabolic Rating: N/A
Androgenic Rating: N/A
Ephedrine References:
[1] Dopamine-mediated actions of ephedrine in the rat substantia nigra. Munhall AC, Johnson SW. (2006). Brain Res. 1069 (1 ): 96-103. PMID 16386715
[2] Ford MD, Delaney KA, Ling LJ, Erickson T, editors. Clinical Toxicology. Philadelphia: WB Saunders; 2001. ISBN 0-7216-5485-1 Research Laboratories; 1996. ISBN 0-911910-12-3
[3] Principles of Addictions and the Law: Applications in Forensic, Mental Health, and Medical Practice. Norman S. Miller (26 February 2010). Academic Press. p. 307. ISBN 978-0-12-496736-6.
[4] Narcotic Culture: A History of Drugs in China. Frank Dikotter (16 April 2004). University of Chicago Press. p. 199. ISBN 978-0-226-14905-9.
[5] Merck Manuals > EPHEDrine Last full review/revision January 2010.





